

Custom Search
|
|

|
||
 and organics. Particulate and lead emissions are submicron size which
contribute to adverse respiratory effects and are at levels often higher
than those from conventional fuel oil combustion. High emission levels can
be attributed to the higher ash and lead concentrations in the waste oil.
Organic emissions also contribute to adverse health affects. However,
organic emissions are often the result of solvent contaminated waste oils.
This can be minimized by segregating the waste oil.
Although particulate and lead emission concentrations tend to be higher
in waste oils, and there is a potential for organic emissions, the
combustion properties of waste oil are not significantly different from
those of conventional fuels and often display the following air emissions:
2.5.1 Particulate Matter. This is the nongaseous portion of the
combustion exhaust consisting of all solid and liquid material (except
water droplets) suspended in the exhaust gases. It is generally defined as
any material that would not pass through a very fine filter. Particulate
matter can be composed of unburned fuel, sulfur compounds, carbon, ash
constituents in the fuel (including many toxic metals), and even
noncombustible airborne dust entering the combustion air system. Current
devices to remove particulate matter include filtration, mechanical
separation, and electrostatic precipitation.
2.5.2 Sulfur Dioxide. SO2 is a nonflammable, colorless gas that can
be "tasted" in concentrations of less than 1 ppm in the air. In higher
concentrations it has a pungent, noxious odor. SO2 and SO3 are formed
in the combustion process when sulfur contained in the fuel combines with
of sulfur, generally referred to as SOx. SO3 is usually no more than
5 percent of the total SOx generated.
Essentially all sulfur contained
in the fuel is converted into SO2 and S03, and is not highly affected
by boiler operating conditions or design. Although regulating the quantity
of sulfur in the fuel is the primary method of controlling SOx emissions,
stack gas scrubbers can also be effective in removing SO2 from the
combustion exhaust.
2.5.3 Nitrogen Oxides. Nitric oxide (NO) and nitrogen dioxide (NO2)
are generated in the combustion process. These compounds are referred to
as NOx. NO is a colorless, odorless gas that is not considered a direct
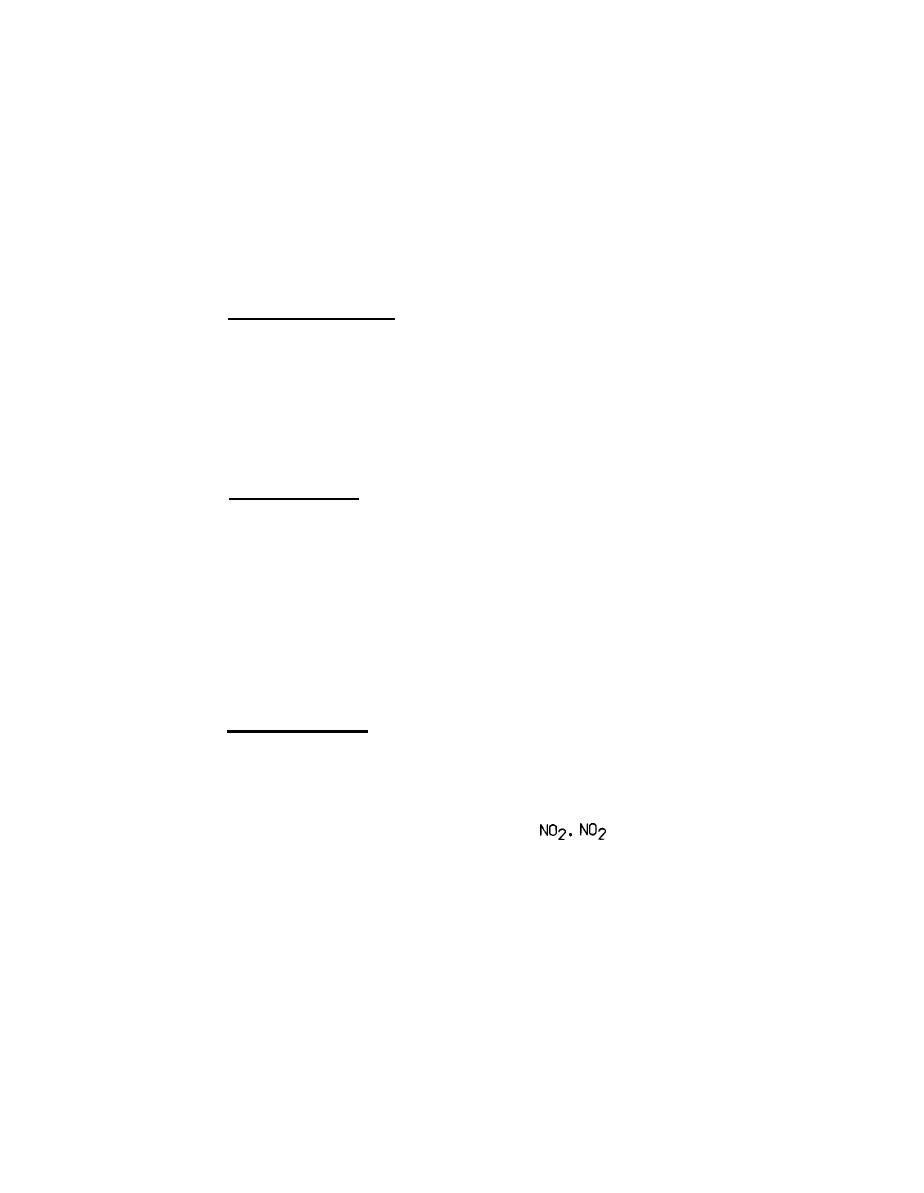
health threat in the concentrations found in the atmosphere. NO2 is
considerably more harmful and comprises typically 5 percent or less of
total NOx emitted from boiler stacks. Once in the atmosphere, however, a
large fraction of the NO is converted into
is a yellow-brown
colored gas having a pungent, sweetish odor. NOx is formed spontaneously
during the combustion process when oxygen and nitrogen are present at high
temperatures. Because all three (oxygen, nitrogen, and high temperatures)
are essential to the combustion process, it would be difficult to prevent
NOx formation.
Nitrogen is present in the combustion air and fuel
itself. Lower peak flame temperatures and reduced excess oxygen, or a
combination of both, have been effective in reducing NOx formation.
the stack.
14
|
 |
|
 |
||